|
|
 |

Vacuum Measurement
Engineers first became interested in vacuum measurements in the 1600s, when they noted the inability of pumps to raise water more than about 30 ft. The Duke of Tuscany in Italy commissioned Galileo to investigate the "problem." Galileo, among others, also devised a number of experiments to investigate the properties of air. Among the tools used for these experiments were pistons to measure force and a water barometer (about 34 ft. tall) to measure vacuum pressure.
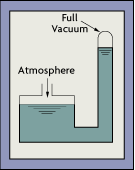 |
Figure 4-3: Barometer
Operation
|
After Galileo's death in 1642, Evangelista Torricelli carried on the work of vacuum-related investigation and invented the mercury barometer (Figure 4-3). He discovered that the atmosphere exerts a force of 14.7 lb. per square in. (psi) and that, inside a fully evacuated tube, the pressure was enough to raise a column of mercury to a height of 29.9 in. (760 mm). The height of a mercury column is therefore a direct measure of the atmospheric pressure.
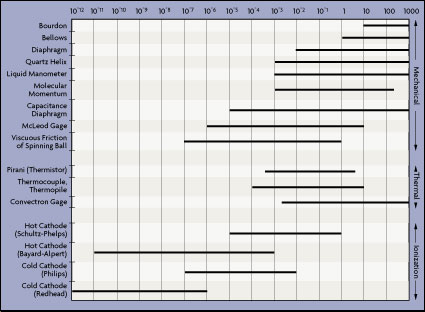 |
| Figure 4-4: Vacuum Gauge Measurement Ranges
|
In 1644, French mathematician Blaise Pascal asked a group of mountaineers to carry a barometer into the Alps and proved that air pressure decreases with altitude. The average barometric pressure at sea level can balance the height of a 760 mm mercury column, and this pressure is defined as a standard Atmosphere. The value for 1/760th of an atmosphere is called a torr, in honor of Torricelli.
In 1872, McLeod invented the McLeod vacuum detector gauge, which measures the pressure of a gas by measuring its volume twice, once at the unknown low pressure and again at a higher reference pressure. The pressurized new volume is then an indication of the initial absolute pressure. Versions of the McLeod Gauge continue to be used today as a standard for calibrating vacuum gauges.
Applications
Vacuum gauges in use today fall into three main categories: mechanical, thermal, and ionization. Their pressure ranges are given in Figure 4-4. In general, for high vacuum services (around 10-6 torr), either cold cathode or Bayard-Alpert hot cathode gauges are suitable. Neither is particularly accurate or stable, and both require frequent calibration.
For vacuums in the millitorr range (required for sputtering applications), one might consider a hot cathode ion gauge. For more accurate measurements in this intermediate range, the capacitance manometer is a good choice. For intermediate vacuum applications (between 10-4 and 10-2 torr), capacitance manometers are the best in terms of performance, but are also the most expensive. The lowest priced gauge is the thermocouple type, but its error is the greatest. Digital Pirani gauges can represent a good compromise solution, with accuracy between that of capacitance and thermocouple sensors.
For low vacuums (higher pressures) between atmospheric and 10-2 torr, Bourdon tubes, bellows, active strain gages, and capacitance sensors are all suitable.
|

