President of the Comité Consultatif de Thermométrie and Vice-President of the Comité International des Poids et Mesures Division of Physics, National Research Council of Canada, Ottawa, K1A OS1 Canada
Received: October 24, 1989
Introductory Note
The official French text of the ITS-90 is published by the BIPM as part of the Prochès-verbaux of the Comité International des Poids et Mesures (CIPM). However, the English version of the text reproduced here has been authorized by the Comité Consultatif de Thermométrie (CCT) and approved by the CIPM.The International Temperature Scale of 1990
The International Temperature Scale of 1990 was adopted by the International Committee of Weights and Measures at its meeting in 1989, in accordance with the request embodied in Resolution 7 of the 18th General Conference of Weights and Measures of 1987. This scale supersedes the International Practical Temperature Scale of 1968 (amended edition of 1975) and the 1976 Provisional 0.5 K to 30 K Temperature Scale.1. Units of Temperature
The unit of the fundamental physical quantity known as thermodynamic temperature, symbol T, is the kelvin symbol K, defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of water1.Because of the way earlier temperature scales were defined, it remains common practice to express a temperature in terms of its difference from 273.15 K, the ice point. A thermodynamic temperature, T, expressed in this way is known as a Celsius temperature, symbol t, defined by:
The unit of Celsius temperature is the degree Celsius, symbol °C, which is by definition equal in magnitude to the kelvin. A difference of temperature may be expressed in kelvins or degrees Celsius.t / °C = T / K - 273.15 (1)
The International Temperature Scale of 1990 (ITS-90) defines both International Kelvin Temperatures, symbol T90, and International Celsius Temperatures, symbol T90. The relation between T90 and T90 is the same as that between T and t, i.e.:
The unit of the physical quantity T90 is the kelvin, symbol K, and the unit of the physical quantity T90 is the degree Celsius, symbol °C, as is the case for the thermodynamic temperature T and the Celsius temperature t.t90 / °C = T90 / K - 273.15 (2)
2. Principles of the International Temperature Scale of 1990 (ITS-90)
The ITS-90 extends upwards from 0.65 K to the highest temperature practicably measurable in terms of the Planck radiation law using monochromatic radiation. The ITS-90 comprises a number of ranges and sub-ranges throughout each of which temperatures T90 are defined. Several of these ranges or sub-ranges overlap, and where such overlapping occurs, differing definitions of T90 exist: these differing definitions have equal status. For measurements of the very highest precision there may be detectable numerical differences between measurements made at the same temperature but in accordance with differing definitions. Similarly, even using one definition, at a temperature between defining fixed points two acceptable interpolating instruments (e.g. resistance thermometers) may give detectably differing numerical values of T90. In virtually all cases these differences are of negligible practical importance and are at the minimum level consistent with a scale of no more than reasonable complexity; for further information on this point see "Supplementary information for the ITS-90" (BIPM-1990).
The ITS-90 has been constructed in such a way that, throughout its range, any given temperature the numerical value of T90 is a close approximation to the numerical value of T90 according to best estimates at the time the scale was adopted. By comparison with direct measurements of thermodynamic temperatures, measurements of T90 are more easily made, are more precise and are highly reproducible.
There are significant numerical differences between the values of T90 and the corresponding values of T90 measured on the International Practical Temperature Scale of 1968 (IPTS-68), see Fig. 1 and Table 6. Similarly there were differences between the IPTS-68 and the International Practical Temperature Scale of 1948 (IPTS-48), and between the International Temperature Scale of 1948 (ITS-48) and the International Temperature Scale of 1927 (ITS-27). See the Appendix, and, for more detailed information, "Supplementary Information for the ITS-90."
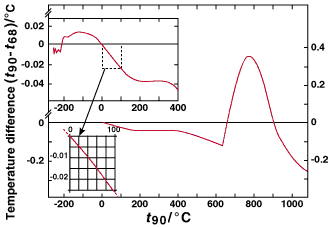
FIG.
1. The differences (t90 - t68) as a function of Celsius temperature
t90.
3. Definition of the International Temperature Scale of 1990
Between
0.65 K and 5.0 K T90 is defined in terms of the vapour-pressure
temperature relations 3He and 4He.
Between
3.0 K and the triple point of neon (24.5561 K) T90 is defined
by means of a helium gas thermometer calibrated at three experimentally
realizable temperatures having assigned numerical values (defining
fixed points) and using specified interpolation procedures.
Between
the triple point of equilibrium hydrogen (13.8033 K) and the freezing
point of silver (961.78 °C) T90 is defined by means of platinum
resistance thermometers calibrated at specified sets of defining
fixed points and using specified interpolation procedures.
Above
the freezing point of silver (961.78°C) T90 is defined in terms
of a defining fixed point and the Planck radiation law.
The
defining fixed points of the ITS-90 are listed in Table 1. The
effects of pressure, arising from significant depths of immersion
of the sensor or from other causes, on the temperature of most
of these points are given in Table 2.
3.1. From 0,65 K: Helium Vapour-Pressure Temperature Equations
In
this range T90 is defined in terms of the vapour pressure
p of 3He and 4He using equations of the
form:
| 9 |
| T90/K = Ao+∑Ai[(in (p/Pa) —B)/C)i |
| i=1 |
The
values of the constants A0, Ai, B and C
are given in Table 3 for 3He in the range of
0.65
K to 3.2 K, and for 4He in the ranges 1.25 K to 2.1768
K (the lambda point) and 2.1768 K to 5.0 K.
3.2 From 3.0 K to the Triple Point of Neon (24.5561 K): Gas Thermometer
In this range T90 is defined in terms of a 3He
or a 4He gas thermometer of the constant-volume type
that has been calibrated at three temperatures. These are the
triple point of neon (24.5561 K), the triple point of equilibrium
hydrogen (13.8033 K), and a temperature is between 3.0 K and 5.0
K. This last temperature is determined using a 3He or a 4He vapour
pressure thermometer as specified in Sect. 3.1.
Table 1. Defining fixed points of the ITS-90
| Temperature | |||||
|---|---|---|---|---|---|
| Number | T90/K | t90/ºC | Substancea | Stateb | Wr(T90) |
| 1 | 3 to 5 | -270.15 to -268.15 |
He | V | |
| 2 | 13.8033 | -259.3467 | e-H2 | T | 0.001 190 07 |
| 3 | ~17 | ~-256.15 | e-H2 (or He) |
V (or G) |
|
| 4 | ~20.3 | ~-252.85 | e-H2 (or He) |
V (or G) |
|
| 5 | 24.5561 | -248.5939 | Ne | T | 0.008 449 74 |
| 6 | 54.3584 | -218.7916 | O2 | T | 0.091 718 04 |
| 7 | 83.8058 | -189.3442 | Ar | T | 0.215 859 75 |
| 8 | 234.3156 | -38.8344 | Hg | T | 0.844 142 11 |
| 9 | 273.16 | 0.01 | H2O | T | 1.000 000 00 |
| 10 | 302.9146 | 29.7646 | Ga | M | 1.118 138 89 |
| 11 | 429.7485 | 156.5985 | In | F | 1.609 801 85 |
| 12 | 505.078 | 231.928 | Sn | F | 1.892 797 68 |
| 13 | 692.677 | 419.527 | Zn | F | 2.568 917 30 |
| 14 | 933.473 | 660.323 | Al | F | 3.376 008 60 |
| 15 | 1234.93 | 961.78 | Ag | F | 4.286 420 53 |
| 16 | 1337.33 | 1064.18 | Au | F | |
| 17 | 1357.77 | 1084.62 | Cu | F | |
(b) For complete definitions and advice on the realization of these various states, see "Supplementary Information for the ITS-90". The symbols have the following meanings: V: vapour pressure point; T: triple point (temperature at which the solid liquid and vapour phases are in equilibrium); G: gas thermometer point; M, F: melting point, freezing point (temperature, at a pressure of 101 325 Pa, at which the solid and liquid phases are in equilibrium)
Table 2. Effect of pressure on the temperatures of some defining fixed points+
| Substance | Assignment value of equilibrium temperature T90/K |
Temperature with pressure, p (dT/dp)/ (10-8K · Pa -1)* |
Variation with depth, lambda (dT/dl)/ (10-3K · m -1)** |
| e-Hydrogen (T) | 13.8033 | 34 | 0.25 |
| Neon (T) | 24.5561 | 16 | 1.9 |
| Oxygen (T) | 54.3584 | 12 | 1.5 |
| Argon (T) | 83.8058 | 25 | 3.3 |
| Mercury (T) | 234.3156 | 5.4 | 7.1 |
| Water (T) | 273.16 | - 7.5 | - 0.73 |
| Gallium | 302.9146 | - 2.0 | - 1.2 |
| Indium | 429.7485 | 4.9 | 3.3 |
| Tin | 505.078 | 3.3 | 2.2 |
| Zinc | 692.677 | 4.3 | 2.7 |
| Aluminium | 933.473 | 7.0 | 1.6 |
| Silver | 1234.93 | 6.0 | 5.4 |
| Gold | 1337.33 | 6.1 | 10 |
| Copper | 1357.77 | 3.3 | 2.6 |
** Equivalent to millikelvins per metre of liquid
+ The Reference pressure for melting and freezing points is the standard atmosphere (p0=101 325 Pa). For triple points (T) the pressure effect is a consequence only of the hydrostatic head of liquid in the cell
Table 3. Values of the constants for the helium vapour pressure Eqs. (3), and the temperature range for which each equation, identified by its set of constants, is valid
| 3He 0.65 K to 3.2 K |
4He 1.25 K to 2.1768 K |
4He 2.1768 K to 5.0 K |
|
|---|---|---|---|
| A0 | 1.053 447 | 1.392 408 | 3.146 631 |
| A1 | 0.980 106 | 0.527 153 | 1.357 655 |
| A2 | 0.676 380 | 0.166 756 | 0.413 923 |
| A3 | 0.372 692 | 0.050 988 | 0.091 159 |
| A4 | 0.151 656 | 0.026 514 | 0.016 349 |
| A5 | - 0.002 263 | 0.001 975 | 0.001 826 |
| A6 | 0.006 596 | - 0.017 976 | - 0.00 4325 |
| A7 | 0.088 966 | 0.005 409 | - 0.00 4973 |
| A8 | - 0.004 770 | 0.013 259 | 0 |
| A9 | - 0.054 943 | 0 | 0 |
| B | 7.3 | 5.6 | 10.3 |
| C | 4.3 | 2.9 | 1.9 |
3.2.1.
From 4.2 K to the Triple Point of Neon (24.5561 K) with 4He
as the Thermometric Gas.
In this range T90 is defined by the relation:
where p is the pressure in the gas thermometer and a, b and c
are coefficients the numerical values of which are obtained from
measurements made at the three defining fixed points given in
Sect. 3.2. but with the further restriction that the lowest one
of these points lies between 4.2 K and 5.0 K.
3.2.2.
From 3.0 K to the Triple Point of Neon (24.5561 K) with 3He
or 4He as the Thermometric Gas.
For
a 3He gas thermometer, and for a 4He gas
thermometer used below 4.2 K, the non-ideality of the gas must
be accounted for explicitly, using the appropriate second virial
coefficient B3 (T90) or B4 (T90). In this
range T90 is defined by the relation:
T90 = a + bp + cp2/1 + Bx(T90) NIV
where
p is the pressure in the gas thermometer, a, b and c are coefficients
the numerical values of which are obtained from measurements at
three defining temperatures as given in Sect. 3.2, N/V is the
gas density with N being the quantity of gas and V the volume
of the bulb, X is 3 or 4 according to the isotope used, and the
values of the second virial coefficients are given by the relations:
For 3He
B(T90)/m3mol-1={16,69 — 336,98(T90/K)-1
+91,04(T90/K)-2—13,82(T90/K)-3} 10-6
For 4He
B4(T90)/m3mol-1={15,708—374,05(T90/K)-1
—383,53(T90/K)-2-2 + 1799,2(T90/K)-3
—4033,2(T90/K)-4 + 3252,8 (T90/K)-3} 10-6
Table
4. The constants A0, Ai; Bn,
Bi; C0, Ci; D0 and
Di in the reference functions of equations (9a); (10a);
and (10b) respectively
| A0 | - 2.135 347 29 | B0 | 0.183 324 722 | C0 | 2.781 572 54 | D0 | 439.932 854 |
| A1 | 3.183 247 20 | B1 | 0.240 975 303 | C1 | 1.646 509 16 | D1 | 472.418 020 |
| A2 | - 1.801 435 97 | B2 | 0.209 108 771 | C2 | - 0.137 143 90 | D2 | 37.684 494 |
| A3 | 0.717 272 04 | B3 | 0.190 439 972 | C3 | - 0.006 497 67 | D3 | 7.472 018 |
| A4 | 0.503 440 27 | B4 | 0.142 648 498 | C4 | - 0.002 344 44 | D4 | 2.920 828 |
| A5 | - 0.618 993 95 | B5 | 0.077 993 465 | C5 | 0.005 118 68 | D5 | 0.005 184 |
| A6 | - 0.053 323 22 | B6 | 0.012 475 611 | C6 | 0.001 879 82 | D6 | - 0.963 864 |
| A7 | 0.280 213 62 | B7 | - 0.032 267 127 | C7 | - 0.002 044 72 | D7 | - 0.188 732 |
| A8 | 0.107 152 24 | B8 | - 0.075 291 522 | C8 | - 0.000 461 22 | D8 | 0.191 203 |
| A9 | - 0.293 028 65 | B9 | - 0.056 470 670 | C9 | 0.000 457 24 | D9 | 0.049 025 |
| A10 | 0.044 598 72 | B10 | 0.076 201 285 | ||||
| A11 | 0.118 686 32 | B11 | - 0.123 893 204 | ||||
| A12 | - 0.052 481 34 | B12 | - 0.029 201 193 | ||||
| B13 | - 0.091 173 542 | ||||||
| B14 | 0.001 317 696 | ||||||
| B15 | 0.026 025 526 |
The
accuracy with which T90 can be realized using Eqs.
(4) and (5) depends on the design of the gas thermometer and the
gas density used. Design criteria and current good practice required
to achieve a selected accuracy are given in "Supplementary Information
for the ITS -90".
3.3. The Triple Point of Equilibrium Hydrogen (13.8033 K) to the Freezing Point of Silver (961.78 °C): Platinum Resistance Thermometer
In
this range T90 is defined by means of a platinum resistance thermometer
calibrated at specified sets of defining fixed points, and using
specified reference and deviation functions for interpolation
at intervening temperatures.
No single platinum resistance thermometer can provide high accuracy,
or is even likely to be usable, over all of the temperature range
13,8033 K to 961.78 °C. The choice of temperature range, or ranges,
from among those listed below for which a particular thermometer
can be used is normally limited by its construction.
For
practical details and current good practice, in particular concerning
types of thermometer available, their acceptable operating ranges,
probable accuracies, permissible leakage resistance, resistance
values, and thermal treatment, see "Supplementary Information
for ITS-90". It is particularly important to take account of the
appropriate heat treatments that should be followed each time
a platinum resistance thermometer is subjected to a temperature
above about 420 °C.
Temperatures
are determined in terms of the ratio of the resistance R(T90)
at a temperature T90 and the resistance R (273.16 K)
at the triple point of water.
This
ratio, W (T90), is 2:
W(T90)=R(T90)/IR(273,16K)
2 Note that this definition of W (T90) differs from the corresponding definition used in the ITS-27, ITS-48, IPTS-48, and IPTS-68: for all of these earlier scales W (T) was defined in terms of reference temperature of 0°C, which since 1954 has itself been defined as 273.15 K
An
acceptable platinum resistance thermometer must be made from pure,
strain-free platinum, and it must satisfy at least one of the
following two relations:
W(27,7646°C)≥1,118,07
W)—38,8344°C)≥0,844 235
An
acceptable platinum resistance thermometer that is to be used
up to the freezing point of silver must also satisfy the relation:
W(961,78°C)≥4,2844
In
each of the resistance thermometer ranges, T90 is obtained
from W (T90) as given by the appropriate reference
function {Eqs. (9b) or (10b)}, and the deviation W(T90)
- Wr(T90). At the defining fixed points this deviation
is obtained directly from the calibration of the thermometer:
at intermediate temperatures it is obtained by means of the appropriate
deviation function {Eqs. (12), (13) and (14)}.
(i)
- For the range 13.8033 K to 273.16 K the following reference
function is defined:
12
(9a.)In [Wr(T90)]=A0 + ∑Ai[In (T90)/273,16K + 1,5/1,5]i
i=1
An inverse fnction, equivalent to Eq.(9a.) to within 0,1 mK, is:
15
(9b.) T90/273,16K = B0 + ∑ Bi[Wr(T90)1/6 —0,65/0,35]i
i=1
The
values of the constants A0, Ai, B0 and Bi are given in Table 4.
A
thermometer may be calibrated for use throughout this range or,
using progressively fewer calibration points, for ranges with
low temperature limits of 24.5561 K, 54.3584 K and 83.8058 K,
all having an upper limit of 273.16 K.
(ii)
- For the range 0 °C to 961.78 °C the following reference function
is defined:
9
(10a.) Wr(T90) = C0 + ∑Ci[T90/K — 754,15/481]i
i=1
An inverse function, equivalent to equation (10a.) to within 0,13 mK is:
9
(10b.) T90/K — 273,15 = D0 + ∑ Di[Wr(T90) — 2,64/1,64]i
i=1
The
values of the constants C0, Ci, D0 and Di are given in Table 4.
A
thermometer may be calibrated for use throughout this range or,
using fewer calibration points, for ranges with upper limits of
660.323 °C, 419.527 °C, 231.928 °C, 156.5985 °C or 29.7646 °C,
all having a lower limit of 0 °C.
(iii)
- A thermometer may be calibrated for use in the range 234.3156
K ( - 38.8344 °C) to 29.7646 °C, the calibration being made at
these temperatures and at the triple point of water. Both reference
functions {Eqs. (9) and (10)} are required to cover this range.
The
defining fixed points and deviation functions for the various
ranges are given below, and in summary from in Table 5.
3.3.1.
The Triple Point of Equilibrium Hydrogen (13.8033 K) to the Triple
Point of Water (273.16 K).
The
thermometer is calibrated at the triple points of equilibrium
hydrogen (13.8033 K), neon (24.5561 K), oxygen (54.3584 K), argon
(83.8058 K), mercury (234.3156 K), and water (273.16 K), and at
two additional temperatures close to 17.0 K and 20.3 K. These
last two may be determined either: by using a gas thermometer
as described in Sect. 3.2, in which case the two temperatures
must lie within the ranges 16.9 K to 17.1 K and 20.2 K to 20.4
K respectively; or by using the vapour pressure-temperature relation
of equilibrium hydrogen, in which case the tow temperatures must
lie within the ranges 17.025 K to 17.045 K and 20.26 K to 20.28
K respectively, with the precise values being determined from
Eqs. (11a) and (11b) respectively:
T90/K - 17.035 = (p/kPa - 33.3213)/13.32 (11a)
T90/K - 20.27 = (p/kPa - 101.292)/30 (11b)
(11a.) T90/K — 17,035 = (p/kPa — 33,3213)/13,32
(11b.) T90/K — 20,27 = (p/kPa — 101,292)/30
The deviation function is3
5
(12.) W(T90) — Wr(T90) = a[W(T90)—1] + b[W(T90)—]2 + ∑ ci[In W(T90)]i+n
i=1
3
This deviation function {and also those of Eqs. (13) and (14)}
may be expressed in terms of Wr rather than W; for
this procedure see "Supplementary Information for ITS-90"
with
values for the coefficients a, b and ci being obtained
from measurements at the defining fixed points and with n = 2.
For
this range and for the sub-ranges 3.3.1.1 to 3.3.1.3 the required
values Wr(T90) are obtained from Eq. (9a)
or from Table 1.
3.3.1.1.
The Triple Point of Neon (24.5561 K) to the Triple Point of Water
(273.16 K).
The
thermometer is calibrated at the triple points of equilibrium
hydrogen (13.8033 K), neon (24.5561 K), oxygen (54.3584 K), argon
(83.8058 K), mercury (234.3156 K) and water (273.16 K).
The deviation function is given by Eq. (12) with values for the
coefficients a, b, c1, c2 and c3
being obtained from measurements at the defining fixed points
and with c4 = c5 = n = 0.
3.3.1.2
The Triple Point of Oxygen (54.3584 K) to the Triple Point of
Water (273.16 K).
The
thermometer is calibrated at the triple points of oxygen (54.3584
K), argon (83.8058 K), mercury (234.3156 K) and water (273.16
K).
Table 5. Deviation functions and calibration points for platinum resistance thermometers in the various ranges in which they define T90
| a. Ranges with an upper limit of 273.16 K | |||
| Section | Lower temperature limit (T/K) |
Deviation functions | Calibration points (see Table 1) |
|---|---|---|---|
| 3.3.1 | 13.8033 | As equation (12), with n=2 | 2-9 |
| 3.3.1.1 | 24.5561 | As for 3.3.1 with c4 = c5 = n = 0 | 2, 5-9 |
| 3.3.1.2 | 54.3584 | As for 3.3.1 with c2 = c3 = c4 = c5 = 0, n = 1 | 6-9 |
| 3.3.1.3 | 83.8058 | a[W (T90) - 1]+b[W (T90) - 1] ln W (T90) | 7-9 |
| b. Ranges with a lower limit of 0 ºC | |||
| Section | Lower temperature limit (t/ºC) |
Deviation functions | Calibration points (see Table 1) |
| 3.3.2* | 961.78 | As equation (14) | 9, 12-15 |
| 3.3.2.1 | 660.323 | As for 3.3.2 with d = 0 | 9, 12 - 14 |
| 3.3.2.2 | 419.527 | As for 3.3.2 with c = d = 0 | 9, 12, 13 |
| 3.3.2.3 | 231.928 | As for 3.3.2 with c = d = 0 | 9, 11, 12 |
| 3.3.2.4 | 156.5982 | As for 3.3.2 with b = c = d = 0 | 9, 11 |
| 3.3.2.5 | 29.7646 | As for 3.3.2 with b = c = d = 0 | 9, 10 |
| c. Range from 234.3156 K ( - 38.8344 ºC) to 29.7646 ºC | |||
| 3.3.3 | As for 3.3.2 with c = d = 0 | 8-10 | |
*
Calibration points 9, 12-14 are used with d = 0 for t90
<= 660.323 °C; the values of a, b and c thus obtained are retained
for t90 => 660.323 °C with d being determined from
calibration point 15
The deviation function is given by Eq. (12) with values for the coefficients a, b and c1 being obtained from measurements at the defining fixed points, with c2 = c3 = c4 = c5 = 0 and with n = 1.
3.3.1.3. The Triple Point of Argon (83.8058 K) to the Triple Point of Water (273.16 K).
The thermometer is calibrated at the triple points of argon (83,8058 K), mercury (234,3156 K) and water (273,16 K).
The deviation function is:
(13.) W(T90) — Wr(T90) = a[W(T90)—1] + b[W(T90)—1] In W(T90)
with
the values of a and b being obtained from measurements at the
defining fixed points.
3.3.2.
From 0 °C to the Freezing Point of Silver (961.78 °C).
The
thermometer is calibrated at the triple point of water (0,01 °C),
and at the freezing points of tin (231.928 °C), zinc (419.527
°C), aluminium (660.323 °C) and silver (961.78 °C).
The
deviation function is:
(14.) W(T90) — Wr(T90) = a[W(T90)—1] + b[W(T90)—1]2 + c[W(T90)—1]3 + d[W(T90)—W(660,323 °C)]2
For
temperatures below the freezing point of aluminium d = 0, with
the values of a, b and c being determined from the measured deviations
from Wr(T90) at the freezing points of tin,
zinc and aluminium. From the freezing point of aluminium to the
freezing point of silver the above values of a, b and c are retained
and the value of d is determined from the measured deviation from
Wr(T90) at the freezing point of silver.
For
this range and for the sub-ranges 3.3.2.1 to 3.3.2.5 the required
values for Wr(T90) are obtained from Eq.
(10a) or from Table 1.
3.3.2.1.
From 0 °C to the Freezing Point of Aluminium (660.323 °C).
The
thermometer is calibrated at the triple point of water (0.01 °C),
and at the freezing points of tin (231.928 °C), zinc (419.527
°C) and aluminium (660.323 °C).
The
deviation function is given by Eq. (14), with the values of a,
b and c being determined from measurements at the defining fixed
points and with d = 0.
3.3.2.2.
From 0 °C to the Freezing Point of Zinc (419.527 °C).
The
thermometer is calibrated at the triple point of water (0.0 °C),
and at the freezing points of tin (231.928 °C). and zinc (419.527
°C).
The
deviation function is given by Eq. (14), with the values of a
and b being obtained from measurements at the defining fixed points
and with c = d = 0.
3.3.2.3.
From 0 °C to the Freezing Point of Tin (231.928 °C).
The
thermometer is calibrated at the triple point of water (0.01 °C),
and at the freezing points of indium (156.5985 °C) and tin (231.928
°C).
The
deviation function is given by Eq. (14), with the values of a
and b being obtained from measurements at the defining fixed points
and with c = d = 0.
3.3.2.4.From
0 °C to the Freezing Point of Indium (156,5985 °C).
The
thermometer is calibrated at the triple point of water (0.01 °C),
and at the freezing point of indium (156.5985 °C).
The
deviation function is given by Eq. (14) with the value of a being
obtained from measurements at the defining fixed points and with
b = c = d = 0.
3.3.2.5.
From 0 °C to the Melting Point of Gallium (29.7646 °C).
The
thermometer is calibrated at the triple point of water (0.01 °C),
and the melting point of gallium (29.7646 °C).
The
deviation function is given by Eq. (14) with the value of a being
obtained from measurements at the defining fixed points and with
b = c = d = 0.
3.3.3.
The Triple Point of Mercury (-38.8344 °C) to the Melting Point
of Gallium (29.7646 °C).
The
thermometer is calibrated at the triple points of mercury (- 38.8344
°C), and water (0.01 °C), and at the melting point of gallium
(29.7646 °C).
The
deviation function is given by Eq. (14) with the values of a and
b being obtained from measurements at the defining fixed points
and with c = d = 0.
The required values of Wr(T90) are obtained
from Eqs. (9a) and (10a) for measurements below and above 273.16
K respectively, or from Table 1.
3.4. The Range Above the Freezing Point of Silver (961,78 °C): Planck Radiation Law
Above
the freezing point of silver the temperature T90 is defined by
the equation:
(15.) Lλ(T90)/Lλ[(T90(X)]=exp(c2[λT90(X)]-1)—1/exp(c2[λT90]-1)—1
where
T90(X) refers to any one of the silver {T90(Ag) = 1234.93
K}, the gold {T90(Au) = 1337.33 K} or the copper {T90(Cu)
= 1357.77 K} freezing points4 and in which Llambda(T90)
and Llambda[T90(X)] are the spectral concentrations
of the radiance of a blackbody at the wavelength (in vacuo) lambda
at T90 and at T90(X) respectively, and c2
= 0.014388 m • K
.
For practical details and current good practice for optical pyrometry,
see "Supplementary Information for the ITS-90" (BIPM-1990).
4
The T90 values of the freezing points of silver, gold
and copper are believed to be self consistent to such a degree
that the substitution of any one of them in place of one of the
other two as the reference temperature T90(X) will
not result in significant differences in the measured values of
T90.
4. Supplementary Information and Differences from Earlier Scales
The
apparatus, methods and procedures that will serve to realize the
ITS-90 are given in "Supplementary Information for the ITS-90".
This document also gives an account of the earlier International
Temperature Scales and the numerical differences between successive
scales that include, where practicable, mathematical functions
for differences T90 - T68. A number of useful approximations
to the ITS-90 are given in "Techniques for Approximating the ITS-90".
These
two documents have been prepared by the Comité Consultatif de
Thermométrie and are published by the BIPM; they are revised and
updated periodically. The differences T90 - T68
are shown in Fig. 1 and Table 6. The number of significant figures
given in Table 6 allows smooth interpolations to be made. However,
the reproducibility of the IPTS-68 is, in many areas, substantially
worse than is implied by this number.
Table 6. Differences between ITS-90 and EPT-76, and between ITS-90 and IPTS-68 for specified values of T90 and t90.
| (T90 - T76)/mK | ||||||||||
| T90/K | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | -0.1 | -0.2 | -0.3 | -0.4 | -0.5 | |||||
| 10 | -0.6 | -0.7 | -0.8 | -1.0 | -1.1 | -1.3 | -1.4 | -1.6 | -1.8 | -2.0 |
| 20 | -2.2 | -2.5 | -2.7 | -3.0 | -3.2 | -3.5 | -3.8 | -4.1 | ||
| (T90 - T68)/K | ||||||||||
| T90/K | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 10 | -0.006 | -0.003 | -0.004 | -0.006 | -0.008 | -0.009 | ||||
| 20 | -0.009 | -0.008 | -0.007 | -0.007 | -0.006 | -0.005 | -0.004 | -0.004 | -0.005 | -0.006 |
| 30 | -0.006 | -0.007 | -0.008 | -0.008 | -0.008 | -0.007 | -0.007 | -0.007 | -0.006 | -0.006 |
| 40 | -0.006 | -0.006 | -0.006 | -0.006 | -0.006 | -0.007 | -0.007 | -0.007 | -0.006 | -0.006 |
| 50 | -0.006 | -0.005 | -0.004 | -0.004 | -0.003 | -0.002 | -0.001 | 0.000 | 0.001 | 0.002 |
| 60 | 0.003 | 0.003 | 0.004 | 0.004 | 0.005 | 0.005 | 0.006 | 0.006 | 0.007 | 0.007 |
| 70 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| 80 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| 90 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.009 | 0.009 | 0.009 |
| T90/K | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| 100 | 0.009 | 0.011 | 0.013 | 0.014 | 0.014 | 0.014 | 0.014 | 0.013 | 0.012 | 0.012 |
| 200 | 0.011 | 0.010 | 0.009 | 0.008 | 0.007 | 0.005 | 0.003 | 0.001 | ||
| (t90 - t68)/ºC | ||||||||||
| t90/ºC | 0 | -10 | -20 | -30 | -40 | -50 | -60 | -70 | -80 | -90 |
| -100 | 0.013 | 0.013 | 0.014 | 0.014 | 0.014 | 0.013 | 0.012 | 0.010 | 0.008 | 0.008 |
| 0 | 0.000 | 0.002 | 0.004 | 0.006 | 0.008 | 0.009 | 0.010 | 0.011 | 0.012 | 0.012 |
| t90/ºC | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| 0 | 0.000 | -0.002 | -0.005 | -0.007 | -0.010 | -0.013 | -0.016 | -0.018 | -0.021 | -0.024 |
| 100 | -0.026 | -0.028 | -0.030 | -0.032 | -0.034 | -0.036 | -0.037 | -0.038 | -0.039 | -0.039 |
| 200 | -0.040 | -0.040 | -0.040 | -0.040 | -0.040 | -0.040 | -0.040 | -0.039 | -0.039 | -0.039 |
| 300 | -0.039 | -0.039 | -0.039 | -0.040 | -0.040 | -0.041 | -0.042 | -0.043 | -0.045 | -0.046 |
| 400 | -0.048 | -0.051 | -0.053 | -0.056 | -0.059 | -0.062 | -0.065 | -0.068 | -0.072 | -0.075 |
| 500 | -0.079 | -0.083 | -0.087 | -0.090 | -0.094 | -0.098 | -0.101 | -0.105 | -0.108 | -0.112 |
| 600 | -0.115 | -0.118 | -0.122 | - 0.125* | -0.08 | -0.03 | 0.02 | 0.06 | 0.11 | 0.16 |
| 700 | 0.20 | 0.24 | 0.28 | 0.31 | 0.33 | 0.35 | 0.36 | 0.36 | 0.36 | 0.35 |
| 800 | 0.34 | 0.32 | 0.29 | 0.25 | 0.22 | 0.18 | 0.14 | 0.10 | 0.06 | 0.03 |
| 900 | -0.01 | -0.03 | -0.06 | -0.08 | -0.10 | -0.12 | -0.14 | -0.16 | -0.17 | -0.18 |
| 1000 | -0.19 | -0.20 | -0.21 | -0.22 | -0.23 | -0.24 | -0.25 | -0.25 | -0.26 | -0.26 |
| t90/ºC | 0 | 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 |
| 1000 | -0.26 | -0.30 | -0.35 | -0.39 | -0.44 | -0.49 | -0.54 | -0.60 | -0.66 | |
| 2000 | -0.72 | -0.79 | -0.85 | -0.93 | -1.00 | -1.07 | -1.15 | -1.24 | -1.32 | -1.41 |
| 3000 | -1.50 | -1.59 | -1.69 | -1.78 | -1.89 | -1.99 | -2.10 | -2.21 | -2.32 | -2.43 |
*
A discontinuity in the first derivative of (t90 - t68)
occurs at a temperature of t90 = 630.6 °C, at which (t90
- t68) = - 0.125 °C
Appendix
The International Temperature Scale of 1927 (ITS-27)
The International Temperature Scale of 1927 was adopted by the seventh General Conference of Weights and Measures to overcome the practical difficulties of the direct realization of thermodynamic temperatures by gas thermometry, and as a universally acceptable replacement for the differing existing national temperature scales. The ITS-27 was formulated so as to allow measurements of temperature to be made precisely and reproducibly, with as close an approximation to thermodynamic temperatures as could be determined at that time. Between the oxygen boiling point and the gold freezing point it was based upon a number of reproducible temperatures, or fixed points, to which numerical values were assigned, and two standard interpolating instruments. Each of these interpolating instruments was calibrated at several of the fixed points, this giving the constants for the interpolating formula in the appropriate temperature range. A platinum resistance thermometer was used for the low part and a platinum rhodium/platinum thermocouple for temperatures above 660 °C. For the region above the gold freezing point, temperatures were defined in terms of the Wien radiation law: in practice, this invariably resulted in the selection of an optical pyrometer as the realizing instrument.The International Temperature Scale of 1948 (ITS-48)
The International Temperature Scale of 1948 was adopted by the ninth General Conference. Changes from the ITS-27 were: the lower limit of platinum resistance thermometer range was changed from -190 °C to the defined oxygen boiling point of -182.97 °C, and the junction of the platinum resistance thermometer range and the thermocouple range became the measured antimony freezing point (about 630 °C) in place 660 °C; the silver freezing point was defined as being 960.8 °C instead of 960.5 °C; the gold freezing point replaced the gold melting point (1063 °C); the Planck radiation law replaced the Wien law; the value assigned to the second radiation constant became 1.438 x 10-2 m • K in place of 1,432 x 10-2 m • K the permitted ranges for the constants of the interpolation formula for the standard resistance thermometer and thermocouple were modified; the limitation on lT for optical pyrometry (lambda•T<3x10-3 m • K) was changed on the requirement that "visible" radiation be used.The International Practical Temperature Scale of 1948 (Amended Edition of 1960) (IPTS-48)
The International Practical Temperature Scale of 1948, amended edition of 1960, was adopted by the eleventh General Conference: the tenth General Conference had already adopted the triple point of water as the sole point defining the kelvin, the unit of thermodynamic temperature. In addition to the introduction of the word "Practical", the modifications to the ITS-48 were: the triple point of water, defined as being 0.01 °C, replaced the freezing point of zinc, defined as being 419.505 °C, became a preferred alternative to the sulphur boiling point (444.6 °C) as a calibration point; the permitted ranges for the constants of the interpolation formulae for the standard resistance thermometer and the thermocouple were further modified; the restriction to "visible" radiation for optical pyrometry was removed.Inasmuch as the numerical values of temperature on the IPTS-48 were the same as on the ITS-48, the former was not a revision of the scale of 1948 but merely an amended form of it.
 CLOSE
CLOSE
